CMS Finalizes Major Changes to Hospital Price Transparency Rule
Hall Render
DECEMBER 4, 2023
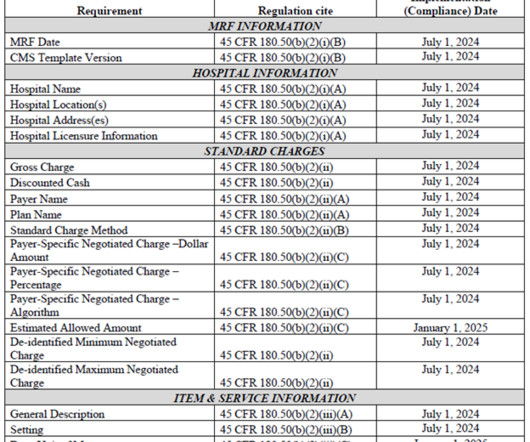
The Centers for Medicare & Medicaid Services (“CMS”) finalized significant updates to the Hospital Price Transparency regulation for the first time since the rule took effect on January 1, 2021.












Let's personalize your content