Five Opportunities to Use the Law to Address Persistent OUD Treatment Gaps
Bill of Health
NOVEMBER 15, 2023
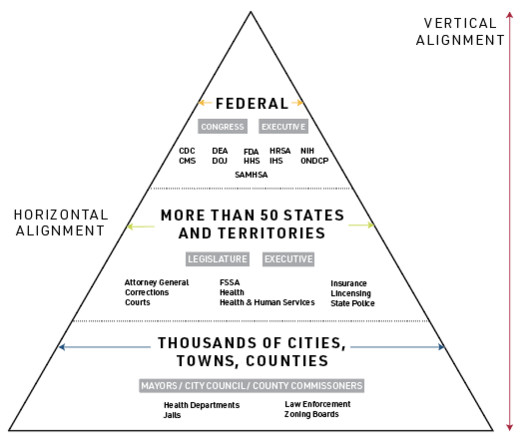
health care system, but that are especially present for behavioral health needs like substance use, and are exacerbated by other challenges related to stigma, lack of employment, and fragmented or nonexistent care coordination. This reality reflects structural, policy, and legal misalignments common to the entire U.S.














Let's personalize your content