Five Opportunities to Use the Law to Address Persistent OUD Treatment Gaps
Bill of Health
NOVEMBER 15, 2023
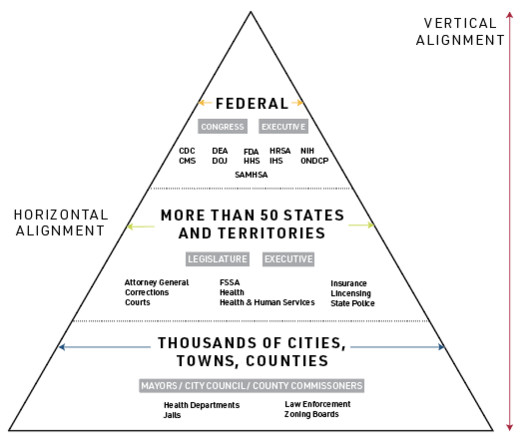
DEA has done little to reduce the appearance of agency capture by the Opioid Treatment Program (OTP) industry, while FDA was years behind the evidence in approving over-the-counter naloxone.










Let's personalize your content