White Paper: Data Optimization: Technology to Support Health Information Workflows
MRO Compliance
NOVEMBER 1, 2022
White Paper: Data Optimization: Technology to Support Health Information Workflows. Download White Paper.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

MRO Compliance
NOVEMBER 1, 2022
White Paper: Data Optimization: Technology to Support Health Information Workflows. Download White Paper.

Healthcare It News
AUGUST 8, 2022
Sponsor: RingCentral Primary topic: Compliance & Legal Topic: Compliance & Legal Patient Engagement Privacy & Security Resource Central: White Papers External url: [link] Thumbnail: Body: Sponsor: RingCentral.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MRO Compliance
MAY 31, 2023
For a decentralized hospital system, there is often a lag time before the ADR even reaches the compliance and audit department. Download White Paper

HIPAA Journal
NOVEMBER 4, 2022
The white paper suggests several areas where policies could be changed to improve cybersecurity in the healthcare industry. Cybersecurity can no longer be viewed as a secondary concern; it must become incorporated into every organization’s – from equipment manufacturers to health care providers – core business models.”.

HealthStream
SEPTEMBER 26, 2019
In this white paper, HCCS—A HealthStream Company, surveyed 281 healthcare compliance leaders throughout the U.S. to learn more about the compliance programs in their hospitals.

Dot Compliance
NOVEMBER 8, 2024
In the pharmaceutical industry, a leading Corrective and Preventive Action (CAPA) process is essential for ensuring product quality and regulatory compliance. Step 3: Risk Assessment Assess the potential impact of the identified problem on product quality, patient safety, and regulatory compliance.

Dot Compliance
JANUARY 14, 2025
Ensuring quality and compliance across an organization continues to be the foundation for effective devices and drug products across the life sciences industry. With an automated solution that eliminates the manual work while ensuring quality and compliance across your business. Whats the best way to do this?

Dot Compliance
JANUARY 29, 2025
Improved Compliance and Risk Management Regulatory compliance: eQMS solutions help organizations meet industry-specific regulations, such as FDA, ISO 9001 , and GMP, by providing built-in compliance features. These features can include automated document version control, audit trail tracking, and built-in compliance templates.

Healthicity
APRIL 23, 2024
Discover the blueprint for compliance education and training straight from the OIG's General Compliance Program Guidance in our exclusive white paper!

YouCompli
JANUARY 28, 2025
If you think about it, healthcare compliance can be comparable to juggling chainsaws in the sense that both require a high level of skill, focus, and precision. Think about your organization as the big top, and all of the responsibilities you have as the compliance officer are your chainsaws!

YouCompli
OCTOBER 9, 2024
Similar to a football coach, a Compliance Officer is accountable to the organizational leadership and Board for the effectiveness of the compliance program. As the Fourth Quarter approaches, compliance program initiatives must be evaluated before the “final whistle” at the end of the year.
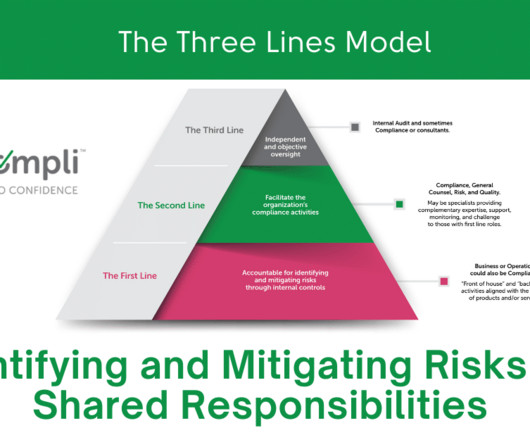
YouCompli
MARCH 1, 2023
Effectively Manage Shared Responsibility A common challenge for compliance officers in healthcare organizations is collaborating with operational leaders to identify and mitigate risks. Often, operational leaders are unaware of their roles and responsibilities in their organizations’ compliance programs. Compliance is the second line.

YouCompli
APRIL 26, 2023
How to create a strong culture and make a valuable impact Compliance officers in healthcare organizations are responsible for building or strengthening culture. The tips below explore how you can convince leaders that a strong compliance culture adds value to the organization. What is a strong culture of compliance in healthcare?

YouCompli
FEBRUARY 15, 2023
Five tips for creating cultural change and showing how healthcare Compliance adds value. Whether it’s “where good ideas go to die” or “the department of no,” compliance officers have likely heard negative descriptions of their department. In compliance, the law and regulations are the basis for everything we do.

Symplr
AUGUST 1, 2022
litigation, operational challenges, and regulatory compliance) is addressed in depth in a white paper that sheds light on the subject. Regulations constantly evolve and differ among states, adding another layer of complexity to an already rigorous process. The topic of such change, dissonance, and its effects (e.g.,

YouCompli
JULY 3, 2023
She says investing in cybersecurity is similar to the challenges of quantifying how Compliance reduces fines. “Yet the likelihood of getting hit by a ransomware attack is much higher than getting hit with an OIG settlement or fine,” Scavotto says. It also underscores the value of liaison programs and operational compliance committees.

Dot Compliance
OCTOBER 25, 2024
In the pharmaceutical industry, it is essential to ensure product quality, patient safety, and regulatory compliance. By combining these two disciplines, organizations can achieve a higher level of quality and compliance. Regulatory Compliance: Demonstrate a robust risk management approach to meet regulatory standards.

Dot Compliance
JUNE 3, 2024
By learning from historical quality data, AI-powered quality systems uncover organization-specific insights, enhancing operational efficiency and compliance. Enact Change Controls Change control processes, essential in life sciences product development and manufacturing, benefit from the AI-based eQMS by meeting regulatory compliance.

YouCompli
MAY 4, 2022
As a compliance officer, you’re integral to achieving these goals. Developing relationships with subject matter experts in these areas is critical to shoring up support for compliance activities across the organization. Charles Mazer, director of Corporate Compliance NewYork-Presbyterian.

YouCompli
MAY 11, 2022
It reflects remarks CJ made at HCCA’s 2022 Compliance Institute. For more insights from the Compliance Institute, download our white paper on how compliance professionals can help healthcare institutions mitigate risk.). Compliance officers should watch for: Follow the money.

Dot Compliance
JANUARY 7, 2025
Electronic Quality Management Systems (eQMS) help meet these demands by streamlining processes, helping to meet compliance, and supporting continuous improvement across the enterprise. Compliance monitoring: Ensure that employees receive necessary training to meet regulatory mandates and maintain competency.

Natalia Mazina
FEBRUARY 22, 2024
Other criteria utilized by PBMs include medication adherence rates, generic compliance ratios, generic effective rate, and medication therapy management implementation. Inmar white paper [link] [link] These are the different “performance” matrix used by PBMs to determine DIR fees. References: [link] [link].

Total Medical Compliance Resources
APRIL 20, 2022
Check out this great white paper on Preventing Workplace Violence: A Road Map for Helathcare. Preparedness is the first step in preventing violence at work. OSHA outlines five core building blocks which when implemented should reduce the chance of a violent event.

YouCompli
MAY 18, 2022
As a compliance officer, you can help your organization cut through the chaos – and mitigate risk. Yet many compliance officers overlook the key to establishing a strong regulatory change management program: building relationships with departmental and operational leaders.

Dot Compliance
OCTOBER 11, 2024
It helps ensure product quality, patient safety, regulatory compliance and more. Regulatory Compliance Adherence to regulatory requirements, such as those outlined by the FDA’s 21 CFR Part 820, is mandatory for pharmaceutical manufacturers. CAPA as a Strategic Tool A good CAPA system is not just a “nice-to-have.”

Dot Compliance
OCTOBER 11, 2024
It helps ensure product quality, patient safety, regulatory compliance and more. Regulatory Compliance Adherence to regulatory requirements, such as those outlined by the FDA’s 21 CFR Part 820, is mandatory for pharmaceutical manufacturers. CAPA as a Strategic Tool A good CAPA system is not just a “nice-to-have.”

MRO Compliance
APRIL 1, 2022
HIM Director’s Guide to Driving New Efficiency in ROI. HIM Director’s Guide to Driving New Efficiency in ROI. Technology Rises to Meet Release of Information Needs. Hospitals continually seek new strategies to streamline operations and boost efficiencies. This is especially true in the wake of COVID-19 and increasing financial distress.

Total HIPAA
MARCH 29, 2023
Cost of TigerText – HIPAA Compliant Text Messaging Solution You must purchase the Enterprise Edition to assure HIPAA compliance. Security and compliance. Zinc signs Business Associate Agreements (BAAs) , demonstrating an ongoing investment in enterprise security, compliance and control.

Dot Compliance
FEBRUARY 23, 2024
The Evolution of Quality Processes Years ago, quality management professionals relied on using manual, paper-based systems for managing their quality processes. Their tasks included documenting quality processes, obtaining signatures from authorized individuals, and storing documents for regulatory compliance.

Dot Compliance
MARCH 7, 2023
Such a tool can help you achieve compliance, improve quality and avoid risk. The cloud QMS solution provider should perform a full validation load of the entire data set before final data migration is performed (see white paper section – The foundation for a seamless cloud migration – for specific details on validation).

Compliancy Group
JANUARY 8, 2024
How to Protect Your Practice from OCR Investigation for a Right of Access Violation Providers seeking to avoid joining the company of the practice above can take a series of sensible measures to ensure compliance with the right of access provision. The post A Deep Dive into 2023 HIPAA Violation Fines appeared first on Compliancy Group.

Healthcare IT Today
JANUARY 12, 2023
Most health IT companies are held to a low standard of evidence of value and proof of ROI through marketing white papers and quantification that is not robust or systematically evaluated, rather than being required to demonstrate value through scientific peer review and journal publication. Florian Otto, CEO and Co-Founder at Cedar.

YouCompli
DECEMBER 18, 2024
Download our Latest White Paper Qualified compliance professionals do the heavy lifting for you, simplifying regulatory change management Our in-house team works tirelessly to monitor U.S. Jerry launches, manages, and sells software and content solutions that simplify complex work.

Digital Health News
MARCH 4, 2025
Compliance with Data Coordination Board standards DCB0129 and DCB0160 is mandated under the Health and Social Care Act 2012. Digital clinical safety is becoming increasingly embedded into organisations. However, a number of challenges are limiting the potential impact of the process.

SQA
OCTOBER 18, 2024
Level 2: When a simplified analysis of the EFRA inspection report is performed to evaluate compliance with the GMP requirements and may be adopted unilaterally by ANVISA. The classification criteria include: Serious deficiencies: Typically linked to non-compliance with critical items. Major deficiencies: Associated with major items.

HIT Consultant
JANUARY 11, 2023
Karen Kobelski, Business Unit Vice President & General Manager, Health, Clinical Surveillance, Compliance & Data Solutions at Wolters Kluwer. In 2023, we expect clinical leaders trying to build solutions will experience pushback from internal legal and regulatory teams challenged to manage the burden of compliance.

Hall Render
FEBRUARY 24, 2023
doctor talks importance of diversity in health care Illinois hospital CEO dismissed amid board investigation Illinois paid sick leave legislation heading to Gov.

C&M Health Law
MARCH 15, 2022
CMS will apply more stringent screening protocols of applicants and will more closely monitor model participants to ensure compliance with beneficiary protections.

YouCompli
DECEMBER 10, 2024
It is also reasonable to expect the new administration to downplay any compliance obligations stemming from the Rule, and perhaps even a total lack of enforcement efforts. Susan is a Certified Internal Auditor (CIA), Certified Healthcare Compliance (CHC), Certified Professional Coder (CPC) and holds a Certification in Risk Management (CRMA).

Drug & Device Law
JULY 31, 2023
It’s an interesting topic; Eric recently wrote a paper on it , and Bexis is putting together a “white paper” for the Product Liability Advisory Council on the same subject. From these exercises we concluded that a 50-state survey on intangibles as “products” for product liability purposes would be both doable and useful.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content