Health Care Paradox: Medicare Penalizes Dozens of Hospitals It Also Gives Five Stars
Kaiser Health News
FEBRUARY 8, 2022
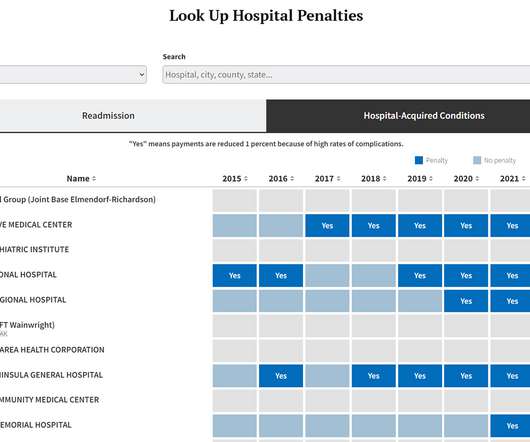
The federal government has penalized 764 hospitals — including more than three dozen it simultaneously rates as among the best in the country — for having the highest numbers of patient infections and potentially avoidable complications. The total amount of the penalties is determined by how much each hospital bills Medicare.











































Let's personalize your content